By Scott E. Olitsky, MD; Errol W. Chan, MBBS, FRCOphth; Sonal Farzavandi, FRCS(Edin)
Accommodative esotropia is defined as a convergent deviation of the eyes associated with activation of the accommodation reflex. It comprises more than 50% of all childhood esotropias1 and can be classified into 3 forms: (1) refractive, (2) non-refractive, and (3) partially accommodative or decompensated.
All 3 forms possess the following characteristics:
- Onset usually between 6 months and 7 years of age, averaging 2.5 years
- Intermittent at onset, then becoming constant over time
- Often initiated by trauma or illness
- Frequently associated with amblyopia
- May be associated with diplopia in older children, but later disappears as a suppression scotoma develops
- Often has a hereditary basis
Refractive Accommodative Esotropia
Pathophysiology
The mechanism involves 3 factors: (1) uncorrected hyperopia, (2) accommodative convergence, and (3) poor fusional divergence. Due to uncorrected high hyperopia, the accommodative drive to produce a clear retinal image leads to increased convergence. If the patient’s fusional divergence is poor and easily overcome, esotropia occurs. Poor fusional divergence may occur if fusional divergence amplitudes are small, or motor fusion is altered by sensory factors. Patients with significant anisometropia are also at risk of developing refractive accommodative esotropia, even though they have lower levels of hyperopia.2
Clinical features
Refractive accommodative esotropia usually occurs after a history of acquired intermittent or constant esotropia. Although this usually occurs in a child between 2 and 3 years of age, children younger than 1 year may sometimes present with all the features of refractive accommodative esotropia. The parents report that the child’s eyes are straight some of the time, but that when the child is focusing at near or is tired, one or both eyes cross inward. At onset, younger children may demonstrate increased eye rubbing or squinting. Older children may complain of asthenopic symptoms such as headaches or diplopia. However, once the esotropia is more manifest and abnormal retinal correspondence develops, these symptoms are relieved.
The average cycloplegic refractive error in refractive accommodative esotropia is +4.75 D,3 but ranges between + 1.5 and +7.0 D. The hypermetropia is predominantly axial in nature.4 Special mention has to be made of the need for accurate cycloplegic refraction, especially in young children, where measurements are often difficult. Cyclopentolate is the standard cycloplegic; however, atropine, which has longer-acting cycloplegic effect, may be required in patients with dark irides to maximize the cycloplegic effect.
The angle of deviation is typically the same for distance and near, averaging between 20 and 40 prism diopters.5 When the esodeviation becomes constant, amblyopia develops.
Treatment
Hyperopic correction
The mainstay of treatment is spectacle correction in refractive accommodative esotropia. The full hyperopic correction based on the cycloplegic refraction is initially prescribed (Figure 1).6 This applies to both juvenile-onset and infantile-onset refractive forms of accommodative esotropia. There is some urgency in initiation of treatment because delay could result in loss of fusion ability, development of amblyopia, and loss of stereopsis.7
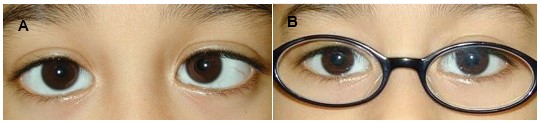
Figure 1. (A) Six-year-old child with refractive accommodative esotropia, hyperopia +4 diopters sphere and a 30 prism diopter esotropia. (B) Prescription of the full cycloplegic hyperopic correction fully corrects the esotropia.
Two important points of parental counseling are pertinent:
- Glasses must be worn on a full-time basis. With part-time use of glasses, the child’s accommodation is never fully relaxed, and vision is blurred whenever the child intermittently returns to using the hyperopic glasses.
- After initiation of spectacle correction, the esotropia will increase when the child is not wearing glasses. Parents often blame the increased esodeviation on initiation of glasses, and thus appropriate counseling is important prior to treatment. This is because after initiation of full-time glasses use, the child becomes accustomed to a much-reduced accommodative effort. However, when the glasses are removed, the child will need to increase accommodative effort to a greater extent than before glasses were prescribed, which will lead to an increased angle of esotropia.
The child is re-evaluated in 1 or 2 months. By definition, if the esotropia is fully corrected within 8 to 10 prism diopters (ie, monofixation range) for near and distance with the full hyperopic correction and the patient regains good fusion, this is consistent with refractive accommodative esotropia, that is, fully accommodative esotropia. Absence of asthenopic symptoms also indicates the success of initial treatment.
If the distance esotropia is still noted to be high, a repeat cycloplegic refraction may be necessary. If there continues to be a significant distance deviation, the patient has a partially accommodative esotropia and may be a candidate for surgery (see Partial or Decompensated Esotropia). If the distance deviation is acceptable and the near esotropia remains high, the patient is considered to have a high accommodative convergence (AC:A) ratio type of accommodative esotropia (see Nonrefractive Accommodative Esotropia).
The long-term natural history of refractive accommodative esotropia is a gradual reduction in hyperopic error.8 When the child is about 9 to 10 years old, the strength of the hyperopic correction can be reduced gradually to enhance fusional divergence and maximize visual acuity. This is usually about 0.50 D to 0.75 D every 12 months. At moderate levels of hyperopia, children may be able to use enough fusional divergence to function without glasses. There is a concern that reducing the hyperopic correction runs the risk of inducing a small esotropia, leading to loss of binocular fusion and amblyopia. Studies have shown that undercorrection of the hyperopia as little as 1.00 D induces decompensation to manifest esotropia in 40% of children.9,10 Therefore, many ophthalmologists wait until the child is older and no longer at risk of amblyopia before starting the weaning process.
Several longitudinal studies have analyzed the long-term outcomes of treatment in refractive accommodative esotropia in terms of amblyopia and stereoacuity. In general, most children with refractive accommodative esotropia develop good binocular potential, although higher-grade stereoacuity may be slightly more limited. This may be related to the age at which binocular disruption occurred, as well as the duration or intermittency of this disruption. In patients who develop the monofixation syndrome, higher-grade stereoacuity cannot be achieved.
- Mulvihill and colleagues reviewed 103 children with refractive accommodative esotropia in Ireland over a mean follow-up period of 4.5 years. At presentation, 61% were amblyopic in one eye, but this decreased to 15% at most recent examination. Stereopsis was observed in 89% of children at the most recent examination.11
- Berk and colleagues reviewed 147 Turkish patients, of whom 59% were amblyopic at presentation. After 5 years of refractive correction, 23% still had 20/40 or worse vision in the amblyopic eye. In addition, although 73% achieved fusion ability, only 24% achieved higher grade stereoacuity between 40 and 100 sec/arc. Children who developed esotropia at a later age (ie, > 2 years) had a higher likelihood of achieving better fusion ability, but this factor was not associated with stereoacuity grade.12
The ability of children to wean off spectacles is more uncertain, with variable results across various studies.
- Lambert and colleagues at Emory University, USA, found that 60% of children with fully accommodative esotropia and baseline refractive errors of +1.50 to +5.00 D were successfully weaned off spectacles starting at a mean age of 8 years. Lower levels of baseline hyperopia of < 3 diopters were associated with higher success rates.13
- However, Mohney and colleagues from the Mayo Clinic, USA, found that the rate of spectacle discontinuation in children with all forms of accommodative esotropia was 8% after 5 years, 20% after 10 years, and 37% after 20 years. Thus, from this study it appears that a majority of children require spectacle correction even into the second decade of life. Factors associated with a lower likelihood of spectacle discontinuation were prematurity and greater hyperopic refractive errors.14
Refractive Surgery
In 1997, Bilgihan reported the first case of a 19-year-old male who was treated with photorefractive keratectomy (PRK) for refractive accommodative esotropia.15 Since then, several authors have investigated the role of PRK and laser in situ keratomileusis (LASIK) for this condition.16-18
A preliminary impression suggests that the role for refractive surgery in refractive accommodative esotropia is likely limited, for 2 reasons. First, refractive errors in this age group are generally not stable; second, the higher hyperopic errors exceed the usual upper limits for hyperopic errors for refractive surgery.
However, the current literature to date suggests that refractive surgery may be fairly successful in treating accommodative esotropia.16-19 Hutchinson and colleagues19 provide a comprehensive review of these various case series. In most cases, the attempted correction was within 0.5 D of cycloplegic hyperopic refractive error. Most cases achieved successful alignment within 10 prism diopters of orthophoria with refractive surgery alone. However, as with corneal refractive surgery procedures, there is always a risk of complications, including corneal haze in the case of PRK, and corneal striae, loss of best corrected visual acuity (BCVA), and diffuse lamellar keratitis for LASIK cases.19
Non-Refractive Accommodative Esotropia
A subgroup of patients with accommodative esotropia have significantly larger esotropia at near, that is, non-refractive or high accommodative convergence (AC:A) ratio accommodative esotropia.
Pathophysiology
The mechanism involves a high AC:A ratio, whereby accommodative effort results in an abnormally high convergence response. Because more accommodation occurs at near, the excess convergence tonus is more manifest at near, so the near esodeviation is larger than that for distance.
Clinical features
Children with high AC:A ratio accommodative esotropia usually present between 2 and 3 years of age. The refractive error in this condition may be hyperopic, emmetropic, or myopic. The average refractive error is +2.25 D.
Measurement of the near angle with accommodative targets is important to demonstrate the full esodeviation. A normal AC:A ratio is 3 to 5 prism diopters: 1 diopter. There are several methods of measuring the AC:A ratio: the heterophoria20 and gradient21 methods, and clinical evaluation of distance and near deviation. The methods of measurement for the heterophoria and gradient methods are as follows:
Gradient Method at 33 cm:
 Heterophoria Method:
Heterophoria Method:
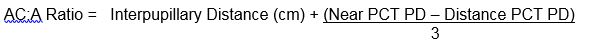
Distance-near Comparison:
Most clinicians prefer to use the distance-near comparison, since this allows the ratio to be evaluated quickly and easily, and it is based on routine examination techniques in the clinic without need for calculations. The AC:A ratio is determined based on the distance and near measurements, whereby if the near esotropia is greater than 10 prism diopters, the AC:A ratio is considered high.
Treatment
To date, there is no consensus on the best treatment for high AC:A ratio accommodative esotropia.
Observation
Some ophthalmologists would observe the excess esotropia at near because it generally improves with age, However, studies to date have not demonstrated a long-term benefit of observation, compared to surgery or bifocals.22,23 Pratt-Johnson and colleagues found that bifocals allow more comfortable binocular fusion for near, but they are not strictly required to preserve overall fusion and stereopsis.22
Bifocal
Bifocals are the most commonly used treatment strategy for non-refractive accommodative esotropia. They are prescribed to relax the near accommodation and correct the near deviation, thus promoting near fusion.24 The bifocals that are often prescribed are the “executive-type” flat-top style that bisect the pupil in primary position. The initial bifocal strength may be estimated based on the near esodeviation, or given arbitrarily as +2.50 D to +3.00 D. Distance refractive correction should be prescribed as appropriate.
When bifocals are prescribed, their full-time use should be indicated to the parents. The child is reviewed after 1 month, and in most cases the esotropia at near responds to bifocal correction. Over time, the bifocal correction can be reduced slowly to increase divergence amplitudes. Reduction of the near add to attain an esophoria of about 4 to 6 prism diopters promotes fusional divergence, but at the same time is sufficient to provide binocular fusion. The target would be to give the child the smallest amount of hyperopic bifocal correction to maintain orthophoria for near fixation.
Miotic agents
In children less than 1 year old, spectacle compliance may not be ideal, owing to their lack of cooperation, their flat nasal bridge, and the difficulty of fitting them appropriately. In this group, anti-accommodative measures include pharmacological measures, such as miotic agents. Miotics are advantageous over bifocals because they allow the child to remain spectacle-free, yet do not require strict cooperation from the child. As with bifocals, miotics have demonstrated fairly consistent results.
Treatment of accommodative esotropia includes echothiopate iodide 0.125%. This agent has a parasympathomimetic effect on the iris sphincter and ciliary muscle, thus reducing the accommodative effort required to obtain a clear retinal image. Consequently, the reflex convergence also reduces.
Echothiophate iodide is administered every evening for 2 weeks, and dosage is gradually lowered as treatment progresses. Parks and colleagues found that this normalizes the AC:A ratio, but the AC:A ratio increases with discontinuation. Ocular side effects include pupil margin cysts, which can be prevented by the use of phenylephrine 2.5% drops. In older children, side effects may include brow ache and miotic spasm. No cases of retinal detachment or angle-closure glaucoma have been noted at these doses in children. However, topical miotics are cholinesterase inhibitors with significant systemic absorption, giving rise to gastrointestinal hypermotility, nausea, and headaches. In addition, echothiophate depletes pseudocholinesterase from the blood, making the patient susceptible to depolarizing muscle relaxants such as succinylcholine, which could prolong respiratory paralysis during anesthesia reversal. In patients on miotics, general anesthesia using non-depolarizing agents is advised.
In general, miotics are considered equivalent to spectacle correction. However, because of the side effect profile of miotics, ophthalmologists generally prefer bifocals to miotics.
Surgery
The role of surgery in non-refractive accommodative esotropia is slightly controversial. It is important to note that the aims of surgery are to normalize the AC:A ratio, to achieve satisfactory alignment, and to allow discontinuation of bifocal use.23,25
There are 2 major types of surgery commonly performed in high AC:A ratio form of accommodative esotropia. O’Hara and Calhoun showed favorable outcomes when operating for the full amount of esotropia at near, although some patients had minimal distance esodeviation.26 Unsurprisingly, there were a few patients who became exotropic for distance. Another technique involves utilizing a posterior fixation, or Faden, suture, in the treatment of these patients.27,28 Here the extent of medial rectus recession is based on the distance angle instead of the near angle, but the Faden suture further controls the higher near esodeviation. Kushner and colleagues found that the augmented surgery technique, targeting the near angle, was more effective in terms of alignment outcomes and discontinuation of bifocal use, compared to that targeting the distance angle and Faden suture placement.29 However, the subject of the best surgical strategy continues to be debated by some surgeons.
More recently, posterior pulley fixation as an alternative to the Faden suture has been described.30 Because no posterior scleral suturing is required, there is a lower risk of scleral perforation.
Partial or Decompensated Accommodative Esotropia
Refractive or non-refractive accommodative esotropias do not always show a reduction in their esodeviations with glasses but have a residual esotropia in spite of full hyperopic correction. These cases have partially accommodative esotropia. Partially accommodative esotropia may also refer to an esotropia that was initially fully accommodative, but that subsequently decompensated over time.31
Risk factors for failure of functional alignment with hyperopic correction only include larger distance deviation, mild hyperopic refractive error, young age at initial prescription, and presence of amblyopia.32 In addition, patients with high AC:A ratio type of accommodative esotropias are more likely to develop a nonaccommodative component requiring surgery.24
Treatment
The initial treatment of partially accommodative esotropia is correction of the full hyperopic error. If after prescribing the full spectacle correction for 4 to 6 weeks, there is residual esotropia of more than 10 prism diopters for distance and near, and the patient is not attaining fusion, there is a general indication for surgery.
Bilateral medial rectus recession is the procedure of choice for partially accommodative esotropia. General indications for surgery include a large angle not fully corrected in spectacles, asthenopic symptoms or diplopia, or cosmetically unacceptable strabismus. However, the extent for surgery is controversial. Some ophthalmologists believe that surgery should aim to correct any deviation to less than 10 prism diopters, because this allows for the development of the monofixation syndrome, which would allow better functional outcomes as peripheral fusion is present. Also, it is thought that the monofixation syndrome would confer better motor fusional vergence, thus preserving the alignment better. On the other hand, other ophthalmologists believe that surgery should only be performed on the nonaccommodative component if it is deemed cosmetically significant by the patient or family. This group does not believe that there is any additional advantage in achieving peripheral fusion.
The following are various formulas for surgical correction of partially accommodative esotropia. In all cases, parents should be advised that surgery would not completely eliminate the use of glasses, but would align the eyes and allow fusion with the use of glasses.
Standard surgery
The standard surgical approach has been to operate for the residual deviation with full hyperopic correction for distance. In high AC:A ratio cases where the near deviation is greater than the distance deviation, operation on the near deviation is acceptable. However, standard surgery has an unacceptably high undercorrection rate of about 25%, and other surgical approaches have been considered.
Wright and colleagues operated on patients using a target angle based on averaging the near deviation with correction and the near deviation without correction. Results comparing augmented surgery to standard surgery showed 93% and 74% success rates, respectively, of reduction to less than 10 prism diopters.33 Patients with high AC:A ratio at baseline had a greater tendency to be overcorrected for distance, with an intermittent exotropia for distance; however, they were all well-aligned for near. This is unsurprising, since the correction is based exclusively on the near angle.
To address this issue for high AC:A ratio cases, a slight modification of the technique by Wright and colleagues involves averaging the near deviation without correction (ie, largest deviation) with the distance deviation without correction (ie, smallest deviation),33 to provide a smaller angle of correction than would have been possible with the augmented surgery formula.
Prism adaptation
Another method involves the use of prism adaptation, which involves prescribing base-out prisms for residual esotropia after full hyperopic correction. The patient then returns in 2 weeks and if the esotropia has increased, a larger prism is given. This process continues at 1- to 2-weekly intervals until the deviation has stabilized. The surgeon then operates on the full “prism-adapted” angle (Figure 2). The Prism Adaptation trial,34 a large multicenter study on prism adaptation, showed that standard surgery resulted in an approximately 75% success rate, compared to 85% with the prism-adapted angle. Prism adaptation has the disadvantages of being more costly and requiring more time.
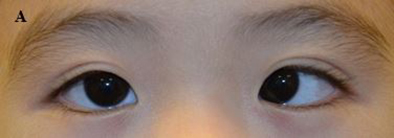
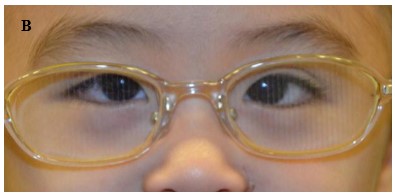
Figure 2. (A) A child with partially accommodative esotropia with hyperopia +3.5Dsph and 50 prism diopters esotropia.; (B) After prescription of the full cycloplegic correction and prism adaptation, there is orthophoria; (C) After bilateral medial rectus recessions for the non-accommodative component of the esotropia, the child is orthophoric.
References
- Rutstein RP. Update on accommodative esotropia. 2008; 79: 422-431.
- Weakley DR Jr, Birch E, Kip K. The role of anisometropia in the development of accommodative esotropia. J AAPOS. 2001; 5:153-157.
- Parks MM. Abnormal accommodative convergence in squint. Arch Ophthalmol. 1958; 59: 364-380.
- Uretmen O, Pamukçu K, Kose S, Egrilmez S. Oculometric features of hyperopia in children with accommodative refractive esotropia. Acta Ophthalmol Scand. 2003; 81: 260-263.
- Preslan MW, Beauchamp GR. Accommodative esotropia: review of current practices and controversies. Ophthalmic Surg. 1987; 18: 68-72.
- Rubin SE. Bringing the management of accommodative esotropia into sharp focus. Am J Ophthalmol. 2006; 141: 914-915.
- Fawcett S, Leffler J, Birch EE. Factors influencing stereoacuity in accommodative esotropia. J AAPOS. 2000; 4: 15-20.
- Park KA, Kim SA, Oh SY. Long-term changes in refractive error in patients with accommodative esotropia. Ophthalmology. 2010; 117: 2196-2207.
- MacEwen CJ, Lymburn EG, Ho WO. Is the maximum hyperopic correction necessary in children with fully accommodative esotropia? Br J Ophthalmol. 2008; 92: 1329-1332.
- Wright KW, Nam E. Different corrections of hypermetropic errors in the successful treatment of hypermetropic amblyopia in children 3 to 7 years of age. Am J Ophthalmol. 2009; 148: 320; author reply -1.
- Mulvihill A, MacCann A, Flitcroft I, O’Keefe M. Outcome in refractive accommodative esotropia. Br J Ophthalmol. 2000; 84: 746-749.
- Berk AT, Koçak N, Ellidokuz H. Treatment outcomes in refractive accommodative esotropia. J AAPOS. 2004; 8: 384-388.
- Lambert SR, Lynn M, Sramek J, Hutcheson KA. Clinical features predictive of successfully weaning from spectacles those children with accommodative esotropia. J AAPOS. 2003; 7: 7-13.
- Mohney BG, Lilley CC, Green-Simms AE, Diehl NN. The long-term follow-up of accommodative esotropia in a population-based cohort of children. 2011; 118: 581-585.
- Bilgihan K, Akata F, Or M, Hasanreisoğlu B. Photorefractive keratectomy in refractive accommodative esotropia. Eye. 1997; 11: 409-410.
- Brugnoli de Pagano OM, Pagano GL. Laser in situ keratomileusis for the treatment of refractive accommodative esotropia. 2012; 119: 159-163.
- Kirwan C, O’Keefe M, O’Mullane GM, Sheehan C. Refractive surgery in patients with accommodative and non-accommodative strabismus: 1-year prospective follow-up. Br J Ophthalmol. 2010; 94: 898-902.
- Magli A, Forte R, Gallo F, Carelli R. Refractive surgery for accommodative esotropia: 5 year follow-up. J Refract Surg. 2014; 30: 116-120.
- Hutchinson AK. Refractive surgery for accommodative esotropia: past, present and future. Eur J Ophthalmol. 2012; 22: 871-877.
- Ansons AM, Davis H. Ocular Deviation. In: Diagnosis and Management of Ocular Motility Disorders. 3rd ed. Oxford: Blackwell Science; 2001: 92-94.
- Rowe FJ. Orthoptic Investigative Procedures. In: Clinical Orthoptics. 3rd ed. Chichester, West Sussex: Wiley-Blackwell.; 2012: 71-72.
- Pratt-Johnson JA, Tillson G. The management of esotropia with high AC/A ratio (convergence excess). J Pediatr Ophthalmol Strabismus. 1985; 22: 238-42.
- Albert DG, Lederman ME. Abnormal distance-near esotropia. Doc Ophthalmol. 1973; 34: 27-36.
- Ludwig IH, Parks MM, Getson RR. Long-term results of bifocal therapy for accommodative esotropia. J Pediatr Ophthalmol Strabismus. 1989; 26: 264-270.
- Rosenbaum AL, Jampolsky A, Scott AB. Bimedial recession in high AC/A esotropia: a long-term follow-up. Arch Ophthalmol. 1974: 91: 251-253.
- O’Hara MA, Calhoun JH. Surgical correction of excess esotropia at near. J Pediatr Ophthalmol Strabismus. 1990; 27: 120-123.
- Millicent M, Peterseim W, Buckley EG. Medial rectus fadenoperation for esotropia only at near fixation. J AAPOS. 1997; 1: 129-133.
- Von Noorden GK, Morris J, Edelman P. Efficacy of bifocals in the treatment of accommodative esotropia. Am J Ophthalmol. 1978; 85: 830-834.
- Kushner BJ, Preslan MW, Morton GV. Treatment of partly accommodative esotropia with a high accommodative convergence-accommodation ratio. Arch Ophthalmol. 1987; 105: 815-818.
- Wabulembo G, Demer JL. Long-term outcome of medial rectus recession and pulley posterior fixation in esotropia with high AC/A ratio. 2012; 20: 115-120.
- Raab EL. Deterioration in accommodative esotropia. In: Reinecke RD, ed. Strabismus II. Orlando: Grune and Stratton, 1984.
- Reddy AK, Freeman CH, Paysse EA, Coats DK. A data-driven approach to the management of accommodative esotropia. Am J Ophthalmol. 2009; 148: 466-470.
- Wright KW, Bruce-Lyle L. Augmented surgery for esotropia associated with high hypermetropia. J Pediatr Ophthalmol Strabismus. 1993; 30:167-170.
- Prism Adaptation Study Research Group. Efficacy of prism adaptation in the surgical management of acquired esotropia. Arch Ophthalmol. 1990; 108: 1248-1256.